Colossal.
A company called Colossal plans on pioneering the de-extinction business, taking species that have died within the past few thousand years and restoring them through the use of DNA editing and stem cells. It’s grabbed headlines recently by announcing some compelling targets: the tylacine, an extinct marsupial predator, and an icon of human carelessness, the dodo. But the company was formed to tackle an even more audacious target: the mammoth, which hasn’t roamed the northern hemisphere for thousands of years.
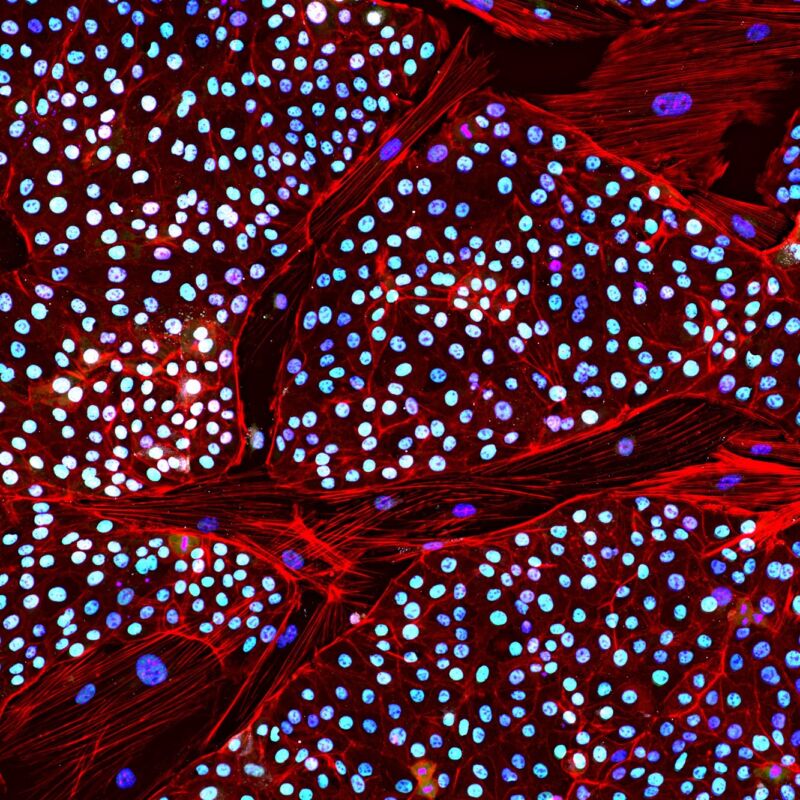
Obviously, there are a host of ethical and conservation issues that would need to be worked out before Colossal’s plans go forward. But there are some major practical hurdles as well, most of them the product of the distinct and extremely slow reproductive biology of the mammoth’s closest living relatives, the elephants. At least one of those has now been cleared, as the company is announcing the production of the first elephant stem cells. The process turned out to be extremely difficult, suggesting that the company still has a long road ahead of it.
Lots of hurdles
Colossal’s basic road map for de-extinction is pretty straightforward. We have already obtained the genomes of a number of species that have gone extinct recently, as well as those of their closest living relatives. By comparing the two, we can identify key genetic differences that make the extinct species distinct. We can then edit those differences into stem cells obtained from the living species and use that species as a surrogate for embryos produced from these stem cells. This will have to be done using stem cells from a number of individuals to ensure that the resulting population has sufficient genetic diversity to be stable.
Most of these technologies have been demonstrated to some extent. We can induce the formation of stem cells from adult cells obtained from several species, do small-scale gene editing, and even produce cloned animals from induced stem cells in a few experimental organisms, like mice.
But that doesn’t mean the process of de-extinction will be like following a recipe from a cookbook. It will be difficult to ensure that we’ve identified all of the key genetic changes that make for a distinct species; editing in only a portion of them might produce inviable organisms. Manipulating bird reproduction requires dealing with the formation of a hard eggshell early in the process, while marsupials have a relatively early birth compared to other mammals, followed by extended gestation in the pouch. And, as we’ve seen many times, things that work just fine in mice don’t always work out when tried on other species.
But the mammoth presents hurdles that are, well… choose your pun from “mammoth” or “colossal.” Elephant gestation time is nearly two years, and they take about 15 years to start breeding. Everything will be slow, and failure late in the process will literally mean the loss of years of work. Nobody has ever made elephant stem cells, and an aquatic period in their evolutionary history has left them with a very complicated reproductive tract. Elephants are also highly intelligent and social, and we have no idea what sort of social environment would need to be provided to make for a viable adult mammoth or whether elephants could provide that.
Overall, it’s a project that has a high probability of failure and may ultimately require generations of scientists. If we do successfully de-extinct a species, the first example will probably be a different species, even though the projects launched later.
If at first you don’t succeed…
But Colossal is forging ahead and cleared one of the many hurdles it faces: It created the first induced stem cells from elephants and will be placing a draft manuscript describing the process on a public repository on Wednesday. (Colossal provided Ars with an advanced version of the draft that, outside of a few editing errors, appears largely complete.) Beyond providing the technical details of how the process works, the manuscript describes a long, failure-ridden route to eventual success:
There are a number of methods that have been developed to allow us to induce stem cells from the cells of an adult organism. The original, Nobel-winning process developed by Shinya Yamanaka involved inserting the genes that encode four key embryonic regulatory genes into adult cells and allowing them to reprogram the adult cell into an embryonic state. That’s proven effective in a variety of species but has a couple of drawbacks due to the fact that the four genes can potentially stick around, interfering with later development steps.
















+ There are no comments
Add yours