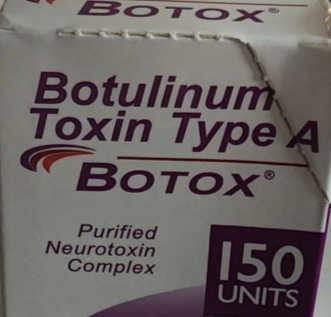
A package of counterfeit Botox.
At least 19 women across nine US states appear to have been poisoned by bogus injections of Botox, the Centers for Disease Control and Prevention reported late Monday.
Nine of the 19 cases—60 percent—were hospitalized and four—21 percent—were treated with botulinum anti-toxin. The CDC’s alert and outbreak investigation follows reports in recent days of botulism-like illnesses linked to shady injections in Tennessee, where officials reported four cases, and Illinois, where there were two. The CDC now reports that the list of affected states also includes: Colorado, Florida, Kentucky, Nebraska, New Jersey, New York, and Washington.
In a separate alert Tuesday, the Food and Drug Administration said that “unsafe, counterfeit” versions of Botox had been found in several states, and the toxic fakes were administered by unlicensed or untrained people and/or in non-medical or unlicensed settings, such as homes or spas. The counterfeit products appeared to have come from an unlicensed source, generally raising the risks that they’re “misbranded, adulterated, counterfeit, contaminated, improperly stored and transported, ineffective and/or unsafe,” the FDA said.
The CDC and the FDA listed the various symptoms that followed injections of the counterfeit Botox, which include: blurred or double vision, drooping eyelids, difficulty swallowing, dry mouth, slurred speech, constipation, incontinence, shortness of breath or difficulty breathing, weakness, and difficulty lifting one’s head. “These symptoms are similar to those seen when botulinum toxin spreads to other parts of the body,” the FDA wrote. Anyone experiencing those symptoms after an injection should go to the emergency room or contact a health care professional.
Botox is a regulated drug containing purified, controlled doses of botulinum toxin, a neurotoxin made by Clostridium bacteria that causes muscle paralysis by blocking a neurotransmitter. It’s often injected into the face to reduce the appearance of wrinkles. The CDC reported that all 19 cases identified so far are in women between the ages of 25 and 59. Eighteen of the 19 specifically reported getting the injections for cosmetic purposes.
But harmful exposure to the toxin—such as from an infection, eating contaminated foods, or use of counterfeit Botox—can cause botulism or at least botulism-like illnesses. In severe cases, botulism can progress to descending, symmetric muscle weakness, full muscle paralysis, and can sometimes be fatal. The CDC reported that some of the people in the outbreak were hospitalized and treated with anti-toxin out of concern that the toxin had spread beyond the injection site. However, the agency noted that five people were specifically tested for botulism, and all tested negative.
In an email to Ars late last week, the CDC recommended that anyone interested in a Botox injection do so using “an FDA-approved product, administered by licensed providers and in licensed settings.” The agency added in its alert Monday: ” If in doubt, don’t get the injection.”
The FDA, meanwhile, provided detailed information on how to ensure your shot of Botox is the real thing. FDA-approved Botox is made by AbbVie, and authentic Botox products come in unit doses of 50, 100, and 200. The outside of the box should say “BOTOX® COSMETIC / onabotulinumtoxinA / for Injection” or “OnabotulinumtoxinA / BOTOX® / for injection,” and it should list the manufacturer as either “Allergan Aesthetics / An AbbVie Company” or “abbvie.” The active ingredient should be listed as “OnabotulinumtoxinA” on the box.
In contrast, some of the counterfeit versions the FDA has tracked down so far were sold in 150-unit doses (not made by AbbVie), only appear to have “Allergan” on the box (not the full manufacturer name), and the active ingredient is displayed as “Botulinum Toxin Type A” instead of “OnabotulinumtoxinA.” The counterfeit versions also have had non-English language text on the outside of the box and displayed a lot number of C3709C3. Any one of these features is a sign that the product is counterfeit. Images of the counterfeit products from the FDA are below.




















+ There are no comments
Add yours